Chapter 4: Matter and Energy in the Universe
Chapter 1
How Science Works
- The Scientific Method
- Evidence
- Measurements
- Units and the Metric System
- Measurement Errors
- Estimation
- Dimensions
- Mass, Length, and Time
- Observations and Uncertainty
- Precision and Significant Figures
- Errors and Statistics
- Scientific Notation
- Ways of Representing Data
- Logic
- Mathematics
- Geometry
- Algebra
- Logarithms
- Testing a Hypothesis
- Case Study of Life on Mars
- Theories
- Systems of Knowledge
- The Culture of Science
- Computer Simulations
- Modern Scientific Research
- The Scope of Astronomy
- Astronomy as a Science
- A Scale Model of Space
- A Scale Model of Time
- Questions
Chapter 2
Early Astronomy
- The Night Sky
- Motions in the Sky
- Navigation
- Constellations and Seasons
- Cause of the Seasons
- The Magnitude System
- Angular Size and Linear Size
- Phases of the Moon
- Eclipses
- Auroras
- Dividing Time
- Solar and Lunar Calendars
- History of Astronomy
- Stonehenge
- Ancient Observatories
- Counting and Measurement
- Astrology
- Greek Astronomy
- Aristotle and Geocentric Cosmology
- Aristarchus and Heliocentric Cosmology
- The Dark Ages
- Arab Astronomy
- Indian Astronomy
- Chinese Astronomy
- Mayan Astronomy
- Questions
Chapter 3
The Copernican Revolution
- Ptolemy and the Geocentric Model
- The Renaissance
- Copernicus and the Heliocentric Model
- Tycho Brahe
- Johannes Kepler
- Elliptical Orbits
- Kepler's Laws
- Galileo Galilei
- The Trial of Galileo
- Isaac Newton
- Newton's Law of Gravity
- The Plurality of Worlds
- The Birth of Modern Science
- Layout of the Solar System
- Scale of the Solar System
- The Idea of Space Exploration
- Orbits
- History of Space Exploration
- Moon Landings
- International Space Station
- Manned versus Robotic Missions
- Commercial Space Flight
- Future of Space Exploration
- Living in Space
- Moon, Mars, and Beyond
- Societies in Space
- Questions
Chapter 5
The Earth-Moon System
- Earth and Moon
- Early Estimates of Earth's Age
- How the Earth Cooled
- Ages Using Radioactivity
- Radioactive Half-Life
- Ages of the Earth and Moon
- Geological Activity
- Internal Structure of the Earth and Moon
- Basic Rock Types
- Layers of the Earth and Moon
- Origin of Water on Earth
- The Evolving Earth
- Plate Tectonics
- Volcanoes
- Geological Processes
- Impact Craters
- The Geological Timescale
- Mass Extinctions
- Evolution and the Cosmic Environment
- Earth's Atmosphere and Oceans
- Weather Circulation
- Environmental Change on Earth
- The Earth-Moon System
- Geological History of the Moon
- Tidal Forces
- Effects of Tidal Forces
- Historical Studies of the Moon
- Lunar Surface
- Ice on the Moon
- Origin of the Moon
- Humans on the Moon
- Questions
Chapter 6
The Terrestrial Planets
- Studying Other Planets
- The Planets
- The Terrestrial Planets
- Mercury
- Mercury's Orbit
- Mercury's Surface
- Venus
- Volcanism on Venus
- Venus and the Greenhouse Effect
- Tectonics on Venus
- Exploring Venus
- Mars in Myth and Legend
- Early Studies of Mars
- Mars Close-Up
- Modern Views of Mars
- Missions to Mars
- Geology of Mars
- Water on Mars
- Polar Caps of Mars
- Climate Change on Mars
- Terraforming Mars
- Life on Mars
- The Moons of Mars
- Martian Meteorites
- Comparative Planetology
- Incidence of Craters
- Counting Craters
- Counting Statistics
- Internal Heat and Geological Activity
- Magnetic Fields of the Terrestrial Planets
- Mountains and Rifts
- Radar Studies of Planetary Surfaces
- Laser Ranging and Altimetry
- Gravity and Atmospheres
- Normal Atmospheric Composition
- The Significance of Oxygen
- Questions
Chapter 7
The Giant Planets and Their Moons
- The Gas Giant Planets
- Atmospheres of the Gas Giant Planets
- Clouds and Weather on Gas Giant Planets
- Internal Structure of the Gas Giant Planets
- Thermal Radiation from Gas Giant Planets
- Life on Gas Giant Planets?
- Why Giant Planets are Giant
- Gas Laws
- Ring Systems of the Giant Planets
- Structure Within Ring Systems
- The Origin of Ring Particles
- The Roche Limit
- Resonance and Harmonics
- Tidal Forces in the Solar System
- Moons of Gas Giant Planets
- Geology of Large Moons
- The Voyager Missions
- Jupiter
- Jupiter's Galilean Moons
- Jupiter's Ganymede
- Jupiter's Europa
- Jupiter's Callisto
- Jupiter's Io
- Volcanoes on Io
- Saturn
- Cassini Mission to Saturn
- Saturn's Titan
- Saturn's Enceladus
- Discovery of Uranus and Neptune
- Uranus
- Uranus' Miranda
- Neptune
- Neptune's Triton
- Pluto
- The Discovery of Pluto
- Pluto as a Dwarf Planet
- Dwarf Planets
- Questions
Chapter 8
Interplanetary Bodies
- Interplanetary Bodies
- Comets
- Early Observations of Comets
- Structure of the Comet Nucleus
- Comet Chemistry
- Oort Cloud and Kuiper Belt
- Kuiper Belt
- Comet Orbits
- Life Story of Comets
- The Largest Kuiper Belt Objects
- Meteors and Meteor Showers
- Gravitational Perturbations
- Asteroids
- Surveys for Earth Crossing Asteroids
- Asteroid Shapes
- Composition of Asteroids
- Introduction to Meteorites
- Origin of Meteorites
- Types of Meteorites
- The Tunguska Event
- The Threat from Space
- Probability and Impacts
- Impact on Jupiter
- Interplanetary Opportunity
- Questions
Chapter 9
Planet Formation and Exoplanets
- Formation of the Solar System
- Early History of the Solar System
- Conservation of Angular Momentum
- Angular Momentum in a Collapsing Cloud
- Helmholtz Contraction
- Safronov and Planet Formation
- Collapse of the Solar Nebula
- Why the Solar System Collapsed
- From Planetesimals to Planets
- Accretion and Solar System Bodies
- Differentiation
- Planetary Magnetic Fields
- The Origin of Satellites
- Solar System Debris and Formation
- Gradual Evolution and a Few Catastrophies
- Chaos and Determinism
- Extrasolar Planets
- Discoveries of Exoplanets
- Doppler Detection of Exoplanets
- Transit Detection of Exoplanets
- The Kepler Mission
- Direct Detection of Exoplanets
- Properties of Exoplanets
- Implications of Exoplanet Surveys
- Future Detection of Exoplanets
- Questions
Chapter 10
Detecting Radiation from Space
- Observing the Universe
- Radiation and the Universe
- The Nature of Light
- The Electromagnetic Spectrum
- Properties of Waves
- Waves and Particles
- How Radiation Travels
- Properties of Electromagnetic Radiation
- The Doppler Effect
- Invisible Radiation
- Thermal Spectra
- The Quantum Theory
- The Uncertainty Principle
- Spectral Lines
- Emission Lines and Bands
- Absorption and Emission Spectra
- Kirchoff's Laws
- Astronomical Detection of Radiation
- The Telescope
- Optical Telescopes
- Optical Detectors
- Adaptive Optics
- Image Processing
- Digital Information
- Radio Telescopes
- Telescopes in Space
- Hubble Space Telescope
- Interferometry
- Collecting Area and Resolution
- Frontier Observatories
- Questions
Chapter 11
Our Sun: The Nearest Star
- The Sun
- The Nearest Star
- Properties of the Sun
- Kelvin and the Sun's Age
- The Sun's Composition
- Energy From Atomic Nuclei
- Mass-Energy Conversion
- Examples of Mass-Energy Conversion
- Energy From Nuclear Fission
- Energy From Nuclear Fusion
- Nuclear Reactions in the Sun
- The Sun's Interior
- Energy Flow in the Sun
- Collisions and Opacity
- Solar Neutrinos
- Solar Oscillations
- The Sun's Atmosphere
- Solar Chromosphere and Corona
- Sunspots
- The Solar Cycle
- The Solar Wind
- Effects of the Sun on the Earth
- Cosmic Energy Sources
- Questions
Chapter 12
Properties of Stars
- Stars
- Star Names
- Star Properties
- The Distance to Stars
- Apparent Brightness
- Absolute Brightness
- Measuring Star Distances
- Stellar Parallax
- Spectra of Stars
- Spectral Classification
- Temperature and Spectral Class
- Stellar Composition
- Stellar Motion
- Stellar Luminosity
- The Size of Stars
- Stefan-Boltzmann Law
- Stellar Mass
- Hydrostatic Equilibrium
- Stellar Classification
- The Hertzsprung-Russell Diagram
- Volume and Brightness Selected Samples
- Stars of Different Sizes
- Understanding the Main Sequence
- Stellar Structure
- Stellar Evolution
- Questions
Chapter 13
Star Birth and Death
- Star Birth and Death
- Understanding Star Birth and Death
- Cosmic Abundance of Elements
- Star Formation
- Molecular Clouds
- Young Stars
- T Tauri Stars
- Mass Limits for Stars
- Brown Dwarfs
- Young Star Clusters
- Cauldron of the Elements
- Main Sequence Stars
- Nuclear Reactions in Main Sequence Stars
- Main Sequence Lifetimes
- Evolved Stars
- Cycles of Star Life and Death
- The Creation of Heavy Elements
- Red Giants
- Horizontal Branch and Asymptotic Giant Branch Stars
- Variable Stars
- Magnetic Stars
- Stellar Mass Loss
- White Dwarfs
- Supernovae
- Seeing the Death of a Star
- Supernova 1987A
- Neutron Stars and Pulsars
- Special Theory of Relativity
- General Theory of Relativity
- Black Holes
- Properties of Black Holes
- Questions
Chapter 14
The Milky Way
- The Distribution of Stars in Space
- Stellar Companions
- Binary Star Systems
- Binary and Multiple Stars
- Mass Transfer in Binaries
- Binaries and Stellar Mass
- Nova and Supernova
- Exotic Binary Systems
- Gamma Ray Bursts
- How Multiple Stars Form
- Environments of Stars
- The Interstellar Medium
- Effects of Interstellar Material on Starlight
- Structure of the Interstellar Medium
- Dust Extinction and Reddening
- Groups of Stars
- Open Star Clusters
- Globular Star Clusters
- Distances to Groups of Stars
- Ages of Groups of Stars
- Layout of the Milky Way
- William Herschel
- Isotropy and Anisotropy
- Mapping the Milky Way
- Questions
Chapter 15
Galaxies
- The Milky Way Galaxy
- Mapping the Galaxy Disk
- Spiral Structure in Galaxies
- Mass of the Milky Way
- Dark Matter in the Milky Way
- Galaxy Mass
- The Galactic Center
- Black Hole in the Galactic Center
- Stellar Populations
- Formation of the Milky Way
- Galaxies
- The Shapley-Curtis Debate
- Edwin Hubble
- Distances to Galaxies
- Classifying Galaxies
- Spiral Galaxies
- Elliptical Galaxies
- Lenticular Galaxies
- Dwarf and Irregular Galaxies
- Overview of Galaxy Structures
- The Local Group
- Light Travel Time
- Galaxy Size and Luminosity
- Mass to Light Ratios
- Dark Matter in Galaxies
- Gravity of Many Bodies
- Galaxy Evolution
- Galaxy Interactions
- Galaxy Formation
- Questions
Chapter 16
The Expanding Universe
- Galaxy Redshifts
- The Expanding Universe
- Cosmological Redshifts
- The Hubble Relation
- Relating Redshift and Distance
- Galaxy Distance Indicators
- Size and Age of the Universe
- The Hubble Constant
- Large Scale Structure
- Galaxy Clustering
- Clusters of Galaxies
- Overview of Large Scale Structure
- Dark Matter on the Largest Scales
- The Most Distant Galaxies
- Black Holes in Nearby Galaxies
- Active Galaxies
- Radio Galaxies
- The Discovery of Quasars
- Quasars
- Types of Gravitational Lensing
- Properties of Quasars
- The Quasar Power Source
- Quasars as Probes of the Universe
- Star Formation History of the Universe
- Expansion History of the Universe
- Questions
Chapter 17
Cosmology
- Cosmology
- Early Cosmologies
- Relativity and Cosmology
- The Big Bang Model
- The Cosmological Principle
- Universal Expansion
- Cosmic Nucleosynthesis
- Cosmic Microwave Background Radiation
- Discovery of the Microwave Background Radiation
- Measuring Space Curvature
- Cosmic Evolution
- Evolution of Structure
- Mean Cosmic Density
- Critical Density
- Dark Matter and Dark Energy
- Age of the Universe
- Precision Cosmology
- The Future of the Contents of the Universe
- Fate of the Universe
- Alternatives to the Big Bang Model
- Space-Time
- Particles and Radiation
- The Very Early Universe
- Mass and Energy in the Early Universe
- Matter and Antimatter
- The Forces of Nature
- Fine-Tuning in Cosmology
- The Anthropic Principle in Cosmology
- String Theory and Cosmology
- The Multiverse
- The Limits of Knowledge
- Questions
Chapter 18
Life On Earth
- Nature of Life
- Chemistry of Life
- Molecules of Life
- The Origin of Life on Earth
- Origin of Complex Molecules
- Miller-Urey Experiment
- Pre-RNA World
- RNA World
- From Molecules to Cells
- Metabolism
- Anaerobes
- Extremophiles
- Thermophiles
- Psychrophiles
- Xerophiles
- Halophiles
- Barophiles
- Acidophiles
- Alkaliphiles
- Radiation Resistant Biology
- Importance of Water for Life
- Hydrothermal Systems
- Silicon Versus Carbon
- DNA and Heredity
- Life as Digital Information
- Synthetic Biology
- Life in a Computer
- Natural Selection
- Tree Of Life
- Evolution and Intelligence
- Culture and Technology
- The Gaia Hypothesis
- Life and the Cosmic Environment
Chapter 19
Life in the Universe
- Life in the Universe
- Astrobiology
- Life Beyond Earth
- Sites for Life
- Complex Molecules in Space
- Life in the Solar System
- Lowell and Canals on Mars
- Implications of Life on Mars
- Extreme Environments in the Solar System
- Rare Earth Hypothesis
- Are We Alone?
- Unidentified Flying Objects or UFOs
- The Search for Extraterrestrial Intelligence
- The Drake Equation
- The History of SETI
- Recent SETI Projects
- Recognizing a Message
- The Best Way to Communicate
- The Fermi Question
- The Anthropic Principle
- Where Are They?
Thermal Radiation
Radiation is the principal way that heat and energy travel through the universe. The energy of each and every star, including the Sun, is carried across space in the form of radiation. With our telescopes on Earth, we capture and analyze that radiation. For now, we will focus on the role of radiation in the transfer of heat and energy.

What basic terms and concepts do we need to talk about radiation? Newton was the first to describe the components of radiation emitted by the Sun. He let a narrow beam of sunlight into a dark room and passed it through a prism. The light spread into the same array of colors that you can see in a rainbow. Newton proved that the visible radiation from the Sun is made up of a mixture of light of all colors. The array of colors that Newton saw — red, orange, yellow, green, blue, indigo, and violet — is called the visible spectrum. (Many people use the mnemonic "Roy G. Biv" to remember this sequence.) Newton was not the first person to disperse light into a spectrum, but he was the first to systematically deduce light's properties. Some scientists suspected that the colors were not part of white light but were introduced by the prism itself. So Newton passed the visible spectrum through a second prism and showed that it recombined back to white light. White light really is a superposition of colors. But are the colors fundamental? Newton selected one color from the spectrum and tried to disperse it further with a second prism. The blue light remained blue light and red light remained red light. The colors, therefore, represent a fundamental property of light.

Newton thought of light as a stream of tiny particles. Other scientists noticed that light had many of the properties of waves. As it turns out, it is equally valid to think of light as a wave or as a particle. In 1800, astronomer and composer William Herschel did an interesting experiment. He passed sunlight through a prism as Newton had done before. When he placed a thermometer in each color, the thermometer heated up, since sunlight of any color carries warming energy. Then he placed the thermometer beyond the red end of the spectrum, where no sunlight is visible. Would it heat up, Herschel wondered? Amazingly, it did. Herschel had discovered that there is radiation "beyond the rainbow" that cannot be detected by our eyes. It is called infrared radiation.
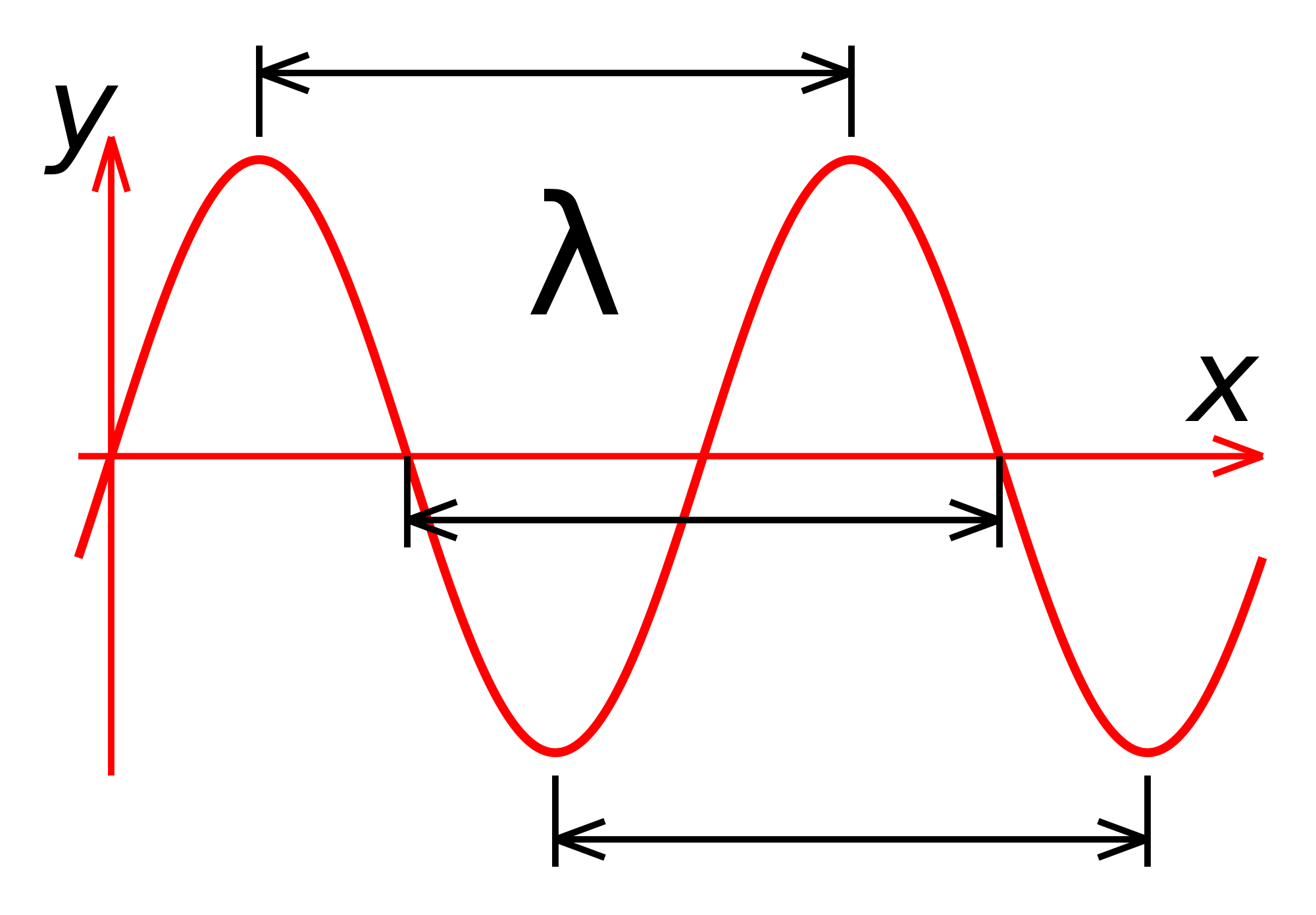
The easiest way to think about radiation is to consider its wave-like qualities. When light is spread out into a spectrum, each color corresponds to a different wavelength. Wavelength refers to the length of the wave — the distance between any two peaks or troughs in the wave. Whenever you see the word "wavelength" in reference to light, you could substitute the word "color" if it helps make the idea clearer. Notice, however, that it is just for convenience that we specify seven colors in the spectrum as listed above. There is actually a smooth and continuous change of color across the spectrum. Similarly, there is a smooth and continuous change of wavelength. Blue light has the shortest wavelength, about 0.0004 millimeters. Red light has longer wavelengths, about 0.0007 millimeters. Infrared radiation has wavelengths that are too long for the eye to see — longer than 0.001 millimeters.

The maximum amount of radiation from the Sun comes in the wavelengths we call yellow: the wavelengths to which our eye's receptor cells are most sensitive. In fact, this is an example of the way that humans adapt to their environment by evolution. The intensity of radiation declines gradually toward longer and shorter wavelengths. From the combination of wavelengths, we see the Sun as yellowish-white. The spectrum of radiation extends beyond the wavelength range to which our eyes are sensitive. Wavelengths too short for our eyes to detect are called ultraviolet radiation. The Sun emits invisible radiation at both ultraviolet and infrared wavelengths.

Temperature is related to the microscopic motions of atoms and molecules. The larger the kinetic energy of the particles, the higher the temperature of the material. Now we see that particles in motion emit a smooth spectrum of radiation. The larger the kinetic energy of the particles, the shorter the peak wavelength of the radiation. The thermal spectrum depends on the temperature in a simple way, given by Wein's law. Since all atoms and molecules are in constant motion, all objects emit thermal radiation. We can also see why the radiation does not depend on composition. If we had a lump of iron and a lump of gold at the same temperature, the iron atoms and the gold atoms have the same kinetic energy. Therefore the iron atoms and the gold atoms emit the same thermal spectrum.
If everything is constantly emitting thermal radiation, why don't we see it? Objects at room temperature emit mainly infrared radiation that we cannot see. Not enough of the radiation comes out in the visible part of the spectrum to be detected by our eyes. We have the technology now to detect and make images with infrared radiation just as we do with visible light. As temperatures increase, the dominant radiation shifts along the spectrum toward bluer or shorter wavelengths. Only when objects reach high temperatures does the dominant radiation move into the visible region of the spectrum. In other words, we can see a radiant glow only from a very hot object.
A good example of Wien's law in action comes when you turn on an electric stove. The coil on the stove starts out at room temperature (about 300 K) and is dull gray. This gray color is not emitted by the coil; it is merely the color of the metal as seen by the ambient light in the room. But then the coil heats up, and eventually, we begin to see a dull red glow. As the coil gets hotter, the glow becomes brighter and eventually appears slightly orange-red. (Molten lava has a similar red glow, and has about the same temperature, about 1100 to 1500 K.) If the coil could get hotter, the radiation would get yellower and finally shift to a mix of colors similar to sunlight, which we perceive as "white" light. Because most objects in daily life are too cool to be "red hot," their thermal radiation is in the infrared, invisible to us.
It is easy to get confused when thinking about color and thermal radiation. We see most ordinary objects by reflected light from the sun or from light bulbs. A blue book is not hotter than a red book; it is just reflecting a different part of the spectrum of a light source. In a room with no light source, a book has no color because there is no light to reflect! We also see the Moon and the planets by reflected sunlight. The only objects that emit their own visible radiation have a temperature of a few thousand Kelvin, like the Sun or the filament of a light bulb. It is important to understand this difference. Now you may be wondering — what about a fluorescent light bulb or tube, which feels cool to the touch? The gas inside this kind of light source has a very low density. So while the gas atoms have high kinetic energy that corresponds to a high temperature, the rate of collisions with the enclosing tube is low so there is little heating effect.
Another type of confusion arises from popular culture. Artists talk about red as a "hot" color and blue as a "cool" color. Musicians use the same terminology — jazz is cool and associated with the color blue and salsa is hot and associated with the color red. Blood is hot and red, but ice is cool and blue. Unfortunately, this subjective description of color is opposite to the scientific description of color based on thermal emission.

